Abstract
Introduction: Multiple myeloma patients over the age of 65 represent the majority of myeloma population. The main goal was to evaluate treatment outcomes in terms of overall survival for elderly patients based on initial choice of anti-myeloma drugs, and to find potential factors affecting survival.
Patients and Methods: This is a retrospective registry based analysis from the Registry of monoclonal gammopathies of the Czech Myeloma Group. Patients with multiple myeloma diagnosed between 2007-2016 over the age of 65 with symptomatic myeloma were included in the analysis. Basic demographic data and disease characteristics were obtained. The Kaplan-Meier estimates were completed by the Greenwood confidence interval. The log-rank test was used to estimate the statistical significance of the difference between the curves. The Cox proportional hazards model was performed to explore the univariate significance of risk factors.
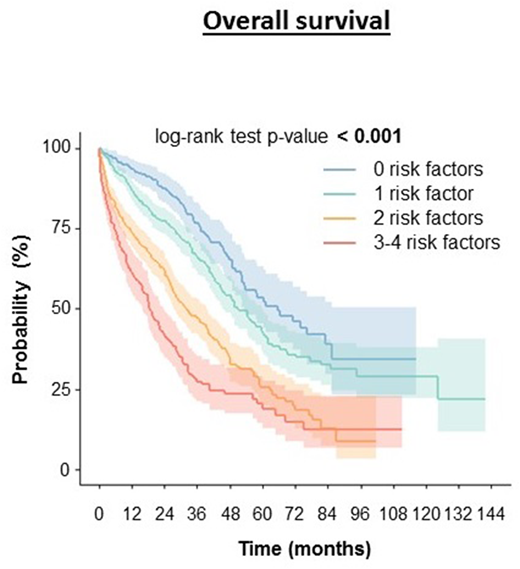
Results: Data from 1410 MM patients were obtained. Gender [HR 1.316 (1.124-1.541), p=0.001], age [above 75 vs. 66-75, HR 1.437 (1.221-1.692), p< 0.001], creatinine levels [at cutoff 152 µmol/L, HR 1.613 (1.365-1.905), p< 0.001] and ECOG performance status [0-1 vs. 2-4, 1.869 (1.594-2.191), p< 0.001] were found to significantly affect overall survival. Moreover these risk factors have cumulative effect on overall survival of the patients. Overall survival of patients regardless to above mentioned risk factors treated with upfront bortezomib (N = 880) was median OS 40.4 months (CI: 36.1-44.7), patients treated with upfront thalidomide (N = 370) had median OS 48.1 months (CI: 41.0-55.2), for lenalidomide (N = 64) median overall survival was 53.2 months (CI: 44.6-61.8) and for combination of bortezomib and thalidomide (N = 46) 32.2 months (CI: 26.6-37.8). When any of these risk factors was present the OS in each group shortened. In the group of patients with no risk factors (N = 255) the median OS for bortezomib (N = 126) was not reached, for thalidomide (N = 96) the median OS was 66.3 months (CI: 43.1-89.6), for lenalidomide (N = 17) 71.1 months (CI: 44.8-97.4) and for combination of bortezomib and thalidomide (N=8) was not reached. In the group of patients with 1 risk factor (N = 514) the median OS for bortezomib (N = 303) was 46.1 months (CI: 36.2-56.1), for thalidomide (N = 141) 56.2 months (CI: 47.5-64.9), for lenalidomide (N = 29) 49.0 months (CI: 9.7-88.2) and for combination of bortezomib and thalidomide (N=20) was not reached. In the group of patients with 2 risk factors (N = 420) the median OS for bortezomib (N = 288) was 34.0 months (CI: 24.7-43.4), for thalidomide (N = 87) 31.9 months (CI: 22.8-40.9), for lenalidomide (N = 14) 33.2 months (CI: 0.0-67.6) and for combination of bortezomib and thalidomide (N=20) 29.4 months (CI: 7.6-51.1). In the group of patients with 3-4 risk factors (N = 221) the median OS for bortezomib (N = 163) was 19.2 months (CI: 14.9-23.5), for thalidomide (N = 46) 18.9 months (CI: 13.0-24.7), for lenalidomide (N = 4) 6.1 months (CI: 0.0-63.0) and for combination of bortezomib and thalidomide (N=3) 14.3 months (CI:-).
Conclusion: The overall survival of patients above the age of 65 shows promising results with the use of novel agents. The treatment outcomes seem to be generally affected by overall condition, age and gender of the patient rather than treatment modality used upfront.
Hajek:Amgen: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Research Funding. Maisnar:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal